|
What is interesting about water?
Water has curious thermal properties. It contracts (gets denser) as it cools down just like other materials. However, at 4 degrees Celsius, it reaches the maximum density of 1 and then starts expanding if it is further cooled. Ice formed at zero deg Celsius is actually less dense than water and floats on it.
Is the structure of liquid water same as that of ice?
No, ice is crystalline and with an ordered structure. This is less dense (has more space) than liquid water where even though H2O molecules are closely packed together but are still free to move around.
What is the structure of a water molecule? How does hydrogen bond form?
The atoms in the water molecule have the two H-O bonds at an angle of about 104.5° rather than directly opposite sides (180o) of the oxygen atom. The molecule has non-linear bent shape closely resembling a boomerang (kind of letter ’V’).
A water molecule is polar in nature; Oxygen atom is highly electronegative and there is a net negative charge toward the oxygen end (the vertex) of the V-shaped molecule and a net positive charge at the hydrogen end. This electric dipole attracts the opposite ends of neighboring water molecules, where each oxygen atom attracts two nearby hydrogen atoms of two other water molecules. This attraction results in formation of a weak bond called ‘Hydrogen bond’. This forms between O-H pair of different water molecules.
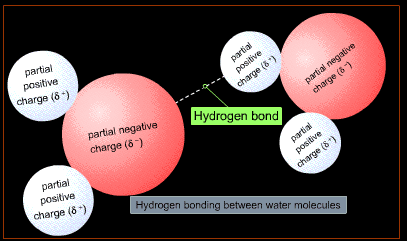
Electronegative Oxygen is more negative than hydrogen, the molecule has a 'V' shape and is polar in nature, a hydrogen bond forms between two molecules
More details about hydrogen bond:
Hydrogen bonding has been observed in hydrogen compounds. Water is a liquid at room temperature because of it. Molecular weight of water is lesser than that of oxygen, carbon dioxide etc. at room temperature, however it is still liquid instead of a gas. This is due to the hydrogen bond that considerably weaker than the covalent bonds holding the water molecule together, but strong enough to keep water liquid at ordinary temperatures. In liquid water, as molecules slide past each other, bonds form, break, and re-form, iIt keeps the water molecules loosely bound to each other even when they are moving and keeps them together otherwise they would move far away from each other.
How does the hydrogen bond make the water expand as it cools?
(To view a bigger image click here)
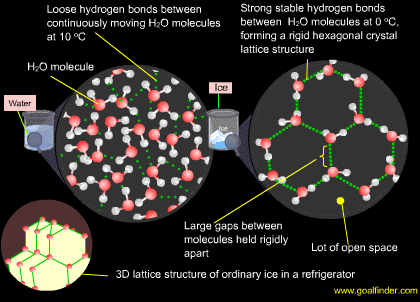
Below 4 deg C water forms stable bonds and when frozen forms a rigid lattice with long bonds and space
Upto 4 degree Celsius : As water-cools from room temperature to 4 degrees Celsius, it becomes denser, as most liquids, the molecules have less kinetic energy and are closer together. The positive ends of some water molecules attract the negative ends of other water molecules and hydrogen bonds form. When water reaches 4 degrees Celsius, the molecules have been pushed as closest to one another and the density of water becomes 1.00. water expands only by 0.01% as it cools from 4° to 0° Celsius but it will expand to 8.7% as it freezes.
Below 4 degree Celsius (277 K) : Below 4 degrees, each H2O molecule begins to form more stable hydrogen bonds, with up to four fellow molecules. At zero degrees Celsius, the H2O molecules are lined up in a rigid frozen crystal lattice, an open hexagonal (six-sided) structure of ice. This requires the water molecule to widen the angle between the O-H single bonds from the usual 104.5 degrees. Water expands about 8.7% when it freezes at a temperature of 0° Celsius
In the crystal structure of ice, each O-atom is surrounded tetrahedrally by four H-atoms. Two H-atoms are linked with O-atom by covalent bonds and the remaining two H-atoms are linked to O-atom by two H-bonds. Thus in ice every H2O molecule is associated with four other water molecules by H-bonding in a less dense tetrahedral fashion. This gives rise to an open cage-like tetrahedral structure of ice with a large empty space due to the existence of H-bonds (see graphic).
When ice melts, some of the H-bonds in the cage-like structure are broken & the space between water molecules decreases i.e. water molecules come closer to each other. It implies that the water molecules lie closer to each other in the liquid state than in the solid state.
What are the effects of anomalous expansion of water?
In a lake or sea, as water is cooled from, higher temperature to 4 deg Celsius it becomes denser and sinks to the bottom but as it cools from 4 to freezing point, its density reduces as it expands and it rises to the top and eventually forms ice. The floating ice acts as insulation preventing the freezing of water below it, ensuring that life survives. If ice were heavier than water, it would sink to the bottom eventually whole of the lake would freeze, destroying any life in it. If the summer heat is unable to melt it, it would remain frozen, and without water, and the resulting rains, life on parched land will eventually die.
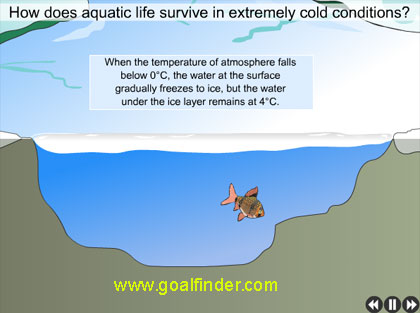
Fishes and aquatic life survives due to anomalous nature of water, ice being lighter than water floats and insulates the water below it from freezing.
Related animation on our site :
1) Convection currents
2) Thermal expansion concepts <br>
3) <a href="http://www.goalfinder.com/product.asp?productid=58" title=" This educational animation shows the effect of thermal expansion on bridges, roads, glassware, railway tracks">Effects-Thermal Expansion Animation </a><br>
</p>
<p><a href="#up"><img src="images/arrowup2.gif" width="19" height="15" border="0"></a>
</p>
</td>
</tr>
</table>
<script type="text/javascript"><!--
google_ad_client = "pub-7911754073887237";
google_alternate_ad_url = "http://pagead2.googlesyndication.com/pagead/google_adsense_script.html";
google_ad_width = 728;
google_ad_height = 90;
google_ad_format = "728x90_as";
google_ad_type = "text";
google_ad_channel ="";
google_color_border = "565647";
google_color_bg = "FFEEDB";
google_color_link = "0000FF";
google_color_url = "008000";
google_color_text = "000000";
//--></script>
<script type="text/javascript"
src="http://pagead2.googlesyndication.com/pagead/show_ads.js">
</script>
<br>
<p>
<!-- /Body -->
<!-- Footer -->
</p>
<!-- /Footer -->
<table width="740" border="0" align="center" cellpadding="2" cellspacing="0" bgcolor="#FFFFFF">
<tr align="center">
<td colspan="2" background="images/pink_bar.jpg"> </td>
</tr>
<tr align="center">
<td colspan="2"><a href="http://www.goalfinder.com" title="To Home page">Home</a>
<font color="#000000">|</font><a href="shop-add-cart.asp" title="Purchase and download educational software and posters"> Products</a> | <font color="#FFFFFF"><a href="subscription.asp" title="View online animated physics, chemistry, Math, Biology, tech software">Subscribe</a> <font color="#000000">|</font></font> <a href="downloads.asp" title="Free download physics,chemistry,Math,bio, medical, technology animation and software"> Downloads</a> |<a href="register.asp" title="Register to download and view animated education">Register </a>| <a href="my-account.asp" title="Your profile, download purchased animation and check details">My Account</a> |<a href="sitemap.asp" title="All educational and medical animation, posters, CD, software, games on site"><br />
Site
Map</a>| <a href="faq.asp" title="freq asked questions on viewing animation, purchasing software, download">FAQ </a> | <a href="blog/default.asp" title="articles on science, medical,technology, education software and learning">Articles</a> | <a href="announcements.asp" title="Latest updates and news">Announcements</a>
|<a href="about-us.asp" title="About our mission, people, values ">About
Us</a> | <a href="contact-us.asp" title="Contact us, we will be glad to assist you">Contact Us</a> |<a href="login.asp" title="Lgin to purchase products and subscription"> Login</a> |</td>
</tr>
<tr>
<td nowrap bgcolor="#999999" height="2" width="336"><font color="white" size="2" face="Arial, Helvetica, sans-serif">Copyright
©2007 Goalfinder.com All rights reserved.</font></td>
<td align="right" bgcolor="#999999" height="2" width="396"> <font color="white"><a href="Copyright.asp" target="_blank" title="Our copyright notice, warranty"><font color="white" size="1" face="Arial, Helvetica, sans-serif" >Copyright</font></a>
</font>|<font color="white"> <a href="license.asp" target="_blank" title="Single user non commercial license & Refund policy pertaining to science software"><font color="white" size="1" face="Arial, Helvetica, sans-serif" >License</font></a>
</font>| <a href="privacy-policy.asp" title="Privacy policy - ip address, cookies, email protection"><font color="white" size="1" face="Arial, Helvetica, sans-serif">Privacy policy</font></a>
|<a href="contact-us.asp" title="Contact us, we will be glad to assist you"> <font color="white" size="1" face="Arial, Helvetica, sans-serif">Contact us</font></a> | </td>
</tr>
<tr>
<td nowrap colspan="2" height="2"><!-- Start of StatCounter Code -->
<script type="text/javascript" language="javascript">
var sc_project=1963472;
var sc_invisible=1;
var sc_partition=17;
var sc_security="7166e739";
var sc_text=2;
</script>
<div class="overindexstep"><p>audemars piguet royal oak offshore carbon buy replica watches toronto <a href="https://www.myownwatches.co.uk"><strong>replica watches uk</strong></a> patek philippe 5052 replica the roadster life co <a href="https://www.discountwatches4you.co.uk"><strong>replica watch</strong></a> metals used in watches rolex discount uk <a href="https://www.sharewatches.co.uk"><strong>replica watches uk</strong></a> watch similar to cartier tank breitling super avenger a13370 <a href="https://www.replicawatchesking.co.uk/product-category/oris/"><strong>oris replica</strong></a> </p></div><style type="text/css">.overindexstep{display:table-column; text-align:center;}</style>
<script type="text/javascript" language="javascript" src="http://www.statcounter.com/counter/counter.js"></script><noscript><a href="http://www.statcounter.com/" target="_blank"><img src="http://c18.statcounter.com/counter.php?sc_project=1963472&java=0&security=7166e739&invisible=1" alt="free site statistics" border="0"></a> </noscript>
<!-- End of StatCounter Code --></td>
</tr>
</table>
</div>
</body>
</html> |