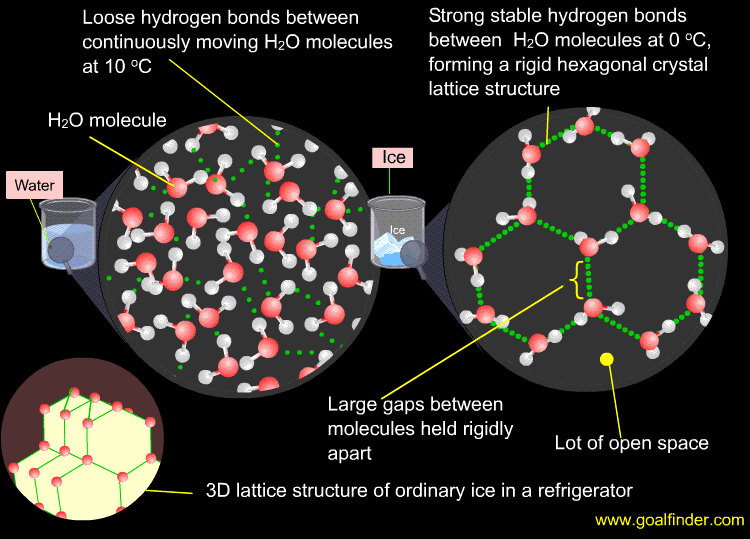
The above image shows the effect of hydrogen bonding causing water to expand in volume ( reduce in density ) as it is cooled below 4 deg celsius to freezing point forming ice. Lot of open space and elongated bonds in a tetrahedral structure ensure an increased volume of ice. A glass bottle filled with water will crack if it is kept in the freezer due to above phenomenon. For more information click here.