|
|
>>
New User Register 

Login Member:
|
|
|
|
| This educational atomic theory animation shows the experiments and foundation work of Goldstein, Wein and Thompson that lead to the discovery of Proton by Rutherford. This 45-minute physics animation depicts in detail the endeavor of the scientists to see the unseen and is meant for high school and college physics classes.
|
Category : Physics
Type : Animation
Animation Type : Advanced
Total animation length: 45 minutes
- Goldstein's canal rays
- Canal rays deflection
- Thomson's contribution
- Nucleus positive core of atom
- Rutherford's proton
- Wilson cloud chamber
- Proton properties
- Proton number
|
|
|
|
|
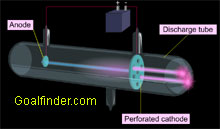
Eugene Goldstein, while carrying out experiments in low-pressure gases, discovered a faint glow behind perforated cathode. He called them canal rays. |
|
|
 |
|
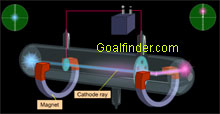
Both Goldstein's discovery of canal rays through perforated cathode and Wein's bending them in magnetic field laid the foundation for discovery of proton. |
|
|
| Experiments carried out by Rutherford by placing source of alpha rays in vacuum and different gases, played a key role in discovering proton. |
|
The hypothesis of Rutherford was confirmed when tracks of proton were seen through the Wilson's cloud chamber. To know more watch preview of this atomic theory animation. |
|
This atomic theory animation explains the following in detail:
- Goldstein's canal rays : Animation shows the steps that lead to the accidental discovery of canal rays by Goldstein and the equipment he used.
- Canal rays deflection : Shown is how Wilhelm Wein contributed by studying the deflection and the apparatus used by him.
- Thomson's contribution : Animated presentation of Thomson study of canal rays and the factors affecting them, included are e/m calculations he performed.
- Nucleus positive core of atom :Around a century back Rutherford had shown nucleus to be the positive core of an atom (refer our animation " Discovery of Nucleus"), an introduction to the gold foil experiment is included here.
- Rutherford's proton : Rutherford discovered Proton a decade after he gold foil experiment, shown are the animation of apparatus, experiments and the step-by-step logic he applied for this.
- Wilson cloud chamber : Brief description of the cloud chamber, its parts and the path of proton.
- Proton properties & Proton Number: Proton properties and how the charge of the nucleus is determined .
|
|
|
|
|
| |
 extensive by amit
extensive by amit |
| |
|
|
|
|
|

