|
|
>>
New User Register 

Login Member:
|
|
|
|
| Mawell Boltzmann's distribution law and graph is completely explained through animation. This 50-minute physics educational animation explains in detail the concept of molecular speed and energy distribution and is meant for high school and college physics classes.
|
Category : Physics
Type : Animation
Animation Type : Advanced
Total animation length: 50 minutes
- Correlation between speed, KE, temperature
- Maxwell's formula
- Understanding distribution graph
- Case1 : Energy distribution and Temperature
- Interactive graph and molecular motion
- Daily life phenomenon - evaporation
- Daily life phenomenon - Chemical Reactions
- Case 2 : Energy distribution and molar mass
- Experimental verification of Maxwell's law
- Mathematical analysis of the experiment
|
|
|
|
|
Animation of experimental verification of Maxwell Boltzmann's distribution law using known concentrations of bismuth vapour and the
velocities at different positions on the glass plate. |
|

|
 |
|
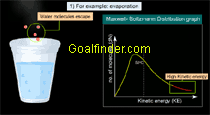
Animated explanation of why do chemical reactions follow distribution law and how evaporation is affected by temperature and why it is a cooling process. |
|
|
|
Maxwell-Boltzmann distribution forms the basis of the kinetic theory of gases, and is useful for explaining molecular speed distribution, gas pressure, diffusion and evaporation of liquid. |
|
This science animation explains the following in detail:
- Correlation between speed, KE, temperature : why does the speed of the molecules in a liquid or gas change continuously
- Maxwell's formula
- Understanding distribution graph : What does area under the curve , low tail, peak, most probable, average, rms speed, speed for lighter molecules
- Case1 : Energy distribution and Temperature :To prove high percentage of molecules will have low energy at low temperatures.
- Interactive graph and molecular motion : Through an interactive graph and increasing motion and collision of molecules understand the significance of graph
- Daily life phenomenon - evaporation : High kinetic energy of the molecules is responsible for the phenomenon of evaporation
- Daily life phenomenon - Chemical Reactions :
- Case 2 : Energy distribution and molar mass : Speed of the molecules is inversely proportional to the molar mass of the molecules
- Experimental verification of Maxwell's law : Experiment explains in details the rotating drum experiment and why spaced patterns of different densities are formed.
- Mathematical analysis of the experiment
Others topics covered are :
- Why collisions cause a change in speed
- Conservation of KE
- Temperature is measure of average KE : importance of using statistical method for determining properties of large number of molecules.
- Why statistical methods are used for large number of molecules
- Why a bell shaped curve is produced?
- Maxwell Boltzmann graph
- most probable, average and rms speeds
- Escaping of hydrogen and helium, effusion & diffusion
- Understanding why particles position varies with speed?
- Explanatory Notes cover :
- Pre Maxwell's Theory
- How Maxwell developed the distribution law ?
|
|
|
|
|
| |
|
|
|
|
|

