Matter can be classified into
pure substances and mixtures.
A Pure substance has uniform composition throughout.
It consist only one phase i.e. it consists of molecule of
only one type
Example: Iron,water, table salt (NaCl) and sugar.
Pure substances are classified into two categories
1) Elements
2) Compounds
Classification of the elements is further done as metals,
nonmetals, and metalloids.
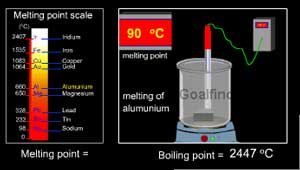
(Metals have a high melting and boiling point)
Compounds, in chemistry, are a substance composed
of atoms of two or more elements in chemical combination,
occurring in a fixed or definite proportion and arranged
in a fixed, definite structure. A compound is often represented
by its chemical formula. The formula for water is H2O,
and for sodium chloride, NaCl. The formula weight of a compound
can be determined from its formula.
Mixtures, in chemistry, are a physical combination
of two or more pure substances (i.e., elements or compounds).
Example: Petrol, milk and air (Air is homogeneous mixture of oxygen, nitrogen, carbon
dioxide and water vapour)
A mixture is distinguished from a compound, which is formed
by the chemical combination of two or more pure substances
in a fixed, definite proportion. The components of a mixture
retain their own chemical properties and may be present
in any proportion. For example, iron filings may be mixed
with powdered sulfur in any proportion, and even if very
fine iron powder is carefully mixed with powdered sulfur,
the two components are easily separated by means of a magnet.
Mixtures are often classified as homogeneous or heterogeneous.
Solutions and colloids are homogeneous mixtures.
The components of a homogeneous mixture are too intimately
combined to be distinguished from one another by visual
observation. A suspension is a heterogeneous mixture. The
particles in a heterogeneous mixture are coarse enough to
be distinguished by visual observation. |

