|
|
>>
New User Register 

Login Member:
|
|
|
|
| This educational atomic theory animation traces the history of discovery of Neutron starting from Bothe and Becker, Rutherford, Joliet and Curie, and ending with Chadwick's discovery of Neutron. This 65-minute physics animation depicts in detail the endeavor of the scientists to see the unseen and is meant for high school and college physics classes.
|
This animation has audio.
Category : Physics
Type : Animation with sound
Animation Type : Advanced
Total animation length: 65 minutes
- Events leading to discovery of neutron
- Experimental discovery of Beryllium Radiation
- Investigation of Beryllium Radiation by Joliet - Curie
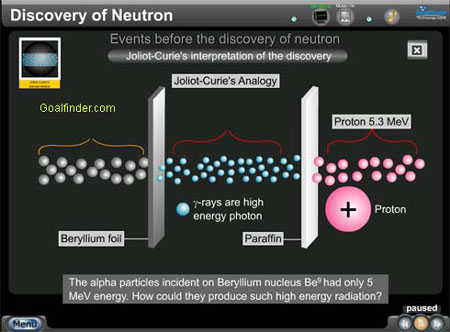
- Joliet -Curie's interpretation of Beryllium radiation
- Discovery of Neutron by Chadwick
- Chadwick's Neutron chamber experiment
- Chadwick's Calculations using Beryllium and Boron
- Explanation of isotopes
- Properties of Neutron
- Quiz on Neutron
Refer details section for more information.
|
|
|
|
|
Discovery of neutron covers the journey in detail thru fully interactive animation with audio. Each icon takes you inside and shows the logic and events that lead to the discovery of neutron in a sequential manner. Our animated mascot, professor Stanley provides you directions and some light moments. |
|
|
|
Each aspect and logic behind the discovery of neutron is looked into. How did Joliet Curie miss the discovery? Why did it take so long to find neutron? How was its mass calculated? Detailed calculations and explanatory tool tips dispel confusion. |
|
|
| Experiments and apparatus used for discovery of neutron are described. Reason behind the Polonium, Beryllium and Paraffin usage and the need for vacuum in ionization chamber, measurement of nitrogen recoil are explained. |
|
 |
A neutron quiz for checking concepts, properties of neutron - mass, location, isotopes, spin, half-life, wavelength, interaction, penetration power and ionization, comparison with x-rays are covered. |
|
This atomic theory animation on neutron is roughly divided into four parts
1) Events leading to discovery of neutron
2) Discovery of neutron by Chadwick
3) Properties of Neutron
4) Quiz
1) Events leading to discovery of neutron :
Discovery of electron and nucleus :
Animation briefly shows contribution of Dalton, J. J Thomson and Rutherford.
Discovery of Proton :
Shows the apparatus and contribution of Thomson and Rutherford in discovering proton
Discovery of Isotopes :
Aston's mass spectrograph and J. J. Thomson's discovery is explained using neon, hydrogen as an example.
What is the missing mass ? :
Scientists were puzzled by the missing mass as protons' mass did not add up to atom's.
Rutherford's prediction of Neutron :
Rutherford predicted presence of neutral particle.
Experimental discovery of Beryllium Radiations :
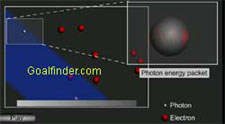
Bothe and Becker are baffled by gamma type of radiations that can penetrate through thick lead.
Investigation of Beryllium Radiation : Joliet - Curie's investigation using polonium, paraffin and ionization chamber. Experimental setup with ionization current levels.
Joliet -Curie's interpretation : Joliet - Curie's interpretation about the mysterious radiation and how Compton effect led them astray.
2) Discovery of neutron by Chadwick
Chadwick's correct interpretation of the experiment :
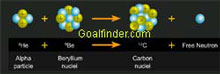
Animation shows Chadwick's logic, experiment with nitrogen, neutral particle theory, alpha particle and Beryllium nuclei.
Neutron chamber experiment of Chadwick :
Velocity measurement of slow moving protons. Estimated calculation using Beryllium and accurate calculation using Boron using energy equation. Explanation of isotopes. Neutron completes the atomic structure.
3) Properties of Neutron
Mass
Location
Isotopes
Spin
Half-life
Wavelength
Interaction
Penetration power
Ionization
Comparison with x-rays
4) Quiz contains questions for checking one's concepts on Neutron.
|
|
|
|
|
| |
|
|
|
|
|

